Athletes, active professionals, and recovery-focused patients increasingly look to the insulin-like growth factor-1 (IGF-1) pathway for muscle and tissue support because it links mechanical loading to protein synthesis and remodelling key drivers of stronger, more resilient bodies. Clinically 1, IGF-1 signalling activates Akt/mTOR, the central switch for translating exercise and nutrition into muscle protein accretion and performance gains.
Your body was designed to grow, repair, and thrive. Vita Bella’s IGF-1 LR3 therapy activates these natural processes, helping you build lean muscle, recover quicker, and sustain peak energy. Experience the smarter, clinical way to restore balance and performance from within with Vita Bella.
How the IGF-1 Pathway Powers Growth?
IGF-1 LR3 is a human IGF-1 analog engineered to bind to IGF-1 receptor activity stronger than its endogenous equivalent it also exhibits weather binding to IGF-binding proteins, thereby improving bioavailability in human systems and cell models; importantly, peer-reviewed 2 work characterizes LR3’s structure/processing and confirms it remains an active IGF-1R ligand.
When you train or rehabilitate after injury, IGF-1 signaling engages satellite cells, boosts translational capacity, and coordinates extracellular matrix remodeling steps required to enlarge existing fibers and support new myofiber formation. Reviews 3 of human muscle biology highlight IGF-1’s role in activating satellite cells and preserving muscle against atrophy, offering a mechanistic rationale for recovery and hypertrophy strategies.
Human preclinical data 4 show direct hypertrophic effects in human myotubes: IGF-1 increased myotube nuclei, fusion index, and myosin heavy chain content, demonstrating true cellular growth responses that underpin strength and size improvements seen with effective training.
Furthermore, IGF-1 5 orchestrates tissue repair beyond fibers; during muscle regeneration, it coordinates with matrix metalloproteinases to mobilize precursor cells and remodel the extracellular matrix processes fundamental to bouncing back from hard sessions or setbacks.
Why is IGF-1 LR3 notable?
LR3 is a variant designed to reduce IGF-binding protein affinity, helping more ligand reach the IGF-1 receptor; analytical studies 6 have even quantified LR3-IGF-1 and related metabolites in human plasma, attesting to its measurable presence and enabling rigorous pharmacological tracking.
Because LR3 maintains receptor activity while improving availability in human-relevant systems, it’s widely studied 7 in human cell culture models to enhance proliferation and survival, providing a translational platform to understand recovery pathways with higher signal through the IGF-1R axis.
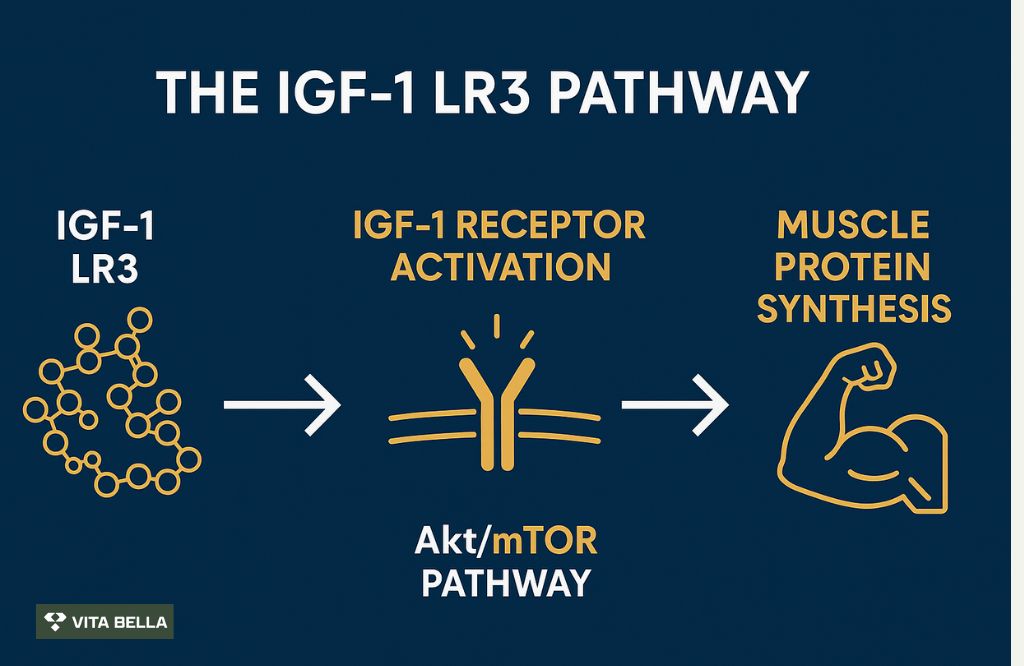
What are the Evidence-based Benefits of IGF-1 LR3?
IGF-1 LR3 appears to enhance muscle growth, accelerate tissue repair, and improve recovery through activation of the Akt/mTOR pathway, a key driver of protein synthesis. It has also shown its ability to increase collagen production, boost cellular energy, and support regenerative healing in muscle and tendon tissues.
1) Muscle Protein Synthesis and Hypertrophy Support
IGF-1 signaling couples training to mTOR activation, increasing muscle protein synthesis ability, an effect consistently reported in human-focused reviews, 1 connecting resistance exercise, nutrient timing, and IGF-1 pathway responsiveness.
2) Cellular Growth in Human Myotubes
In human cell models 4, IGF-1 directly drives hypertrophy markers (greater nuclei per myotube, higher fusion index, elevated contractile protein content), reinforcing its role in the cellular foundation of strength and size gains.
3) Regeneration and Remodeling
IGF-1 coordinates immune-matrix interactions during repair, activating satellite cells and shaping matrix turnover mechanisms necessary for efficient regeneration after intense training or deconditioning.
4) Tendon and Connective-Tissue Support
A human-focused translational review 8 reports IGF-1 stimulates collagen expression and matrix synthesis in human tendon cell–derived constructs, supporting the concept of stronger, better-organized connective tissue during rehabilitation.
5) Anti-Atrophy Protection in Human Contexts
IGF-1 signaling counteracts pathways linked to muscle loss (e.g., ubiquitin–proteasome activity), while enhancing satellite cell activity key for maintaining lean mass during reduced activity or aging in human settings.
6) Pro-Regenerative Crosstalk
Human-centric reviews 9 detail how IGF-1 interacts with cytokines (e.g., IL-6) and niche signals to regulate repair dynamics, offering a systems-level explanation for why restoring IGF-1 signaling aligns with better recovery trajectories.
Unleash Your Potential - Try Vita Bella Today, Build Strength, and Recover Faster!
When your body can’t keep up with your goals, it’s time for smarter recovery. Vita Bella’s IGF-1 LR3 treatment restores optimal growth factor activity to repair tissue, boost energy, and shorten recovery time. Empower your progress and feel unstoppable with precision-driven hormone support.
Backed by cutting-edge research, Vita Bella’s IGF-1 LR3 therapy works in harmony with your body’s natural biology to enhance muscle strength and resilience. Reclaim your edge, recover faster, and experience the power of science-led restoration that keeps you performing at your peak.
FAQs
Is IGF-1 LR3 effective for promoting muscle growth?
Yes, IGF-1 LR3 activates the same receptor pathways responsible for muscle hypertrophy and protein synthesis, including the Akt/mTOR cascade. Human and human-cell studies show it increases myotube nuclei, myosin content, and anabolic signaling, supporting faster muscle recovery and growth.
Does IGF-1 LR3 help improve recovery after intense training?
Yes, IGF-1 LR3 enhances regeneration by stimulating satellite cells and collagen production in human muscle and tendon models. It promotes tissue remodeling, reduces recovery time, and helps maintain muscle integrity after stress or injury.
Is IGF-1 LR3 safe when used under medical supervision?
Yes, when evaluated in controlled preclinical human systems, IGF-1 LR3 maintains receptor specificity and predictable pharmacokinetics. As with any growth-factor therapy, clinical oversight is crucial to ensure balanced dosing and to avoid overstimulation of growth pathways.
Can IGF-1 LR3 replace natural recovery methods like rest and nutrition?
No, IGF-1 LR3 works best when combined with fundamental recovery pillars sleep, protein-rich nutrition, and structured training. It amplifies natural repair mechanisms but should not substitute evidence-based lifestyle practices essential for sustainable growth and health.
References:
Barclay, R. D., Burd, N. A., Tyler, C., Tillin, N. A., & Mackenzie, R. W. (2019). The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Frontiers in Nutrition, 6, 146. https://doi.org/10.3389/fnut.2019.00146
Yang, Y., Wu, J., & Watson, J. T. (2005). Probing the folding pathways of long R3 insulin-like growth factor-I (LR3IGF-I) and IGF-I via capture and identification of disulfide intermediates by cyanylation methodology and mass spectrometry. Biochemistry, 44(27), 8970–8982. https://doi.org/10.1021/bi050232p
Yoshida, T., & Delafontaine, P. (2020). Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells, 9(9), 1970. https://doi.org/10.3390/cells9091970
Jacquemin, V., Furling, D., Bigot, A., Butler-Browne, G. S., & Mouly, V. (2004). IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Experimental Cell Research, 297(1), 148–160. https://doi.org/10.1016/j.yexcr.2004.03.035
Kok, H. J., & Barton, E. R. (2021). Actions and interactions of IGF-I and MMPs during muscle regeneration. Seminars in Cell & Developmental Biology, 119, 48–56. https://doi.org/10.1016/j.semcdb.2021.04.015
Thomas, A., Walpurgis, K., Delahaut, P., Fichant, E., Schänzer, W., & Thevis, M. (2010). Determination of LongR3-IGF-I, R3-IGF-I, Des1–3 IGF-I and their metabolites in human plasma samples by means of LC–MS. Journal of Chromatography B, 878(19), 1562–1568. https://doi.org/10.1016/j.jchromb.2010.04.026
Voorhamme, D., & Yandell, C. A. (2006). LONG R3IGF-I as a more potent alternative to insulin in serum-free culture of HEK293 cells. Molecular Biotechnology, 34(2), 201–204. https://doi.org/10.1385/mb:34:2:201
Miescher, I., Rieber, J., Calcagni, M., & Buschmann, J. (2023). In vitro and in vivo effects of IGF-1 delivery strategies on tendon healing: A review. International Journal of Molecular Sciences, 24(3), 2370. https://doi.org/10.3390/ijms24032370
Forcina, L., Miano, C., Scicchitano, B. M., & Musarò, A. (2019). Signals from the niche: Insights into the role of IGF-1 and IL-6 in modulating skeletal muscle fibrosis. Cells, 8(3), 232. https://doi.org/10.3390/cells8030232